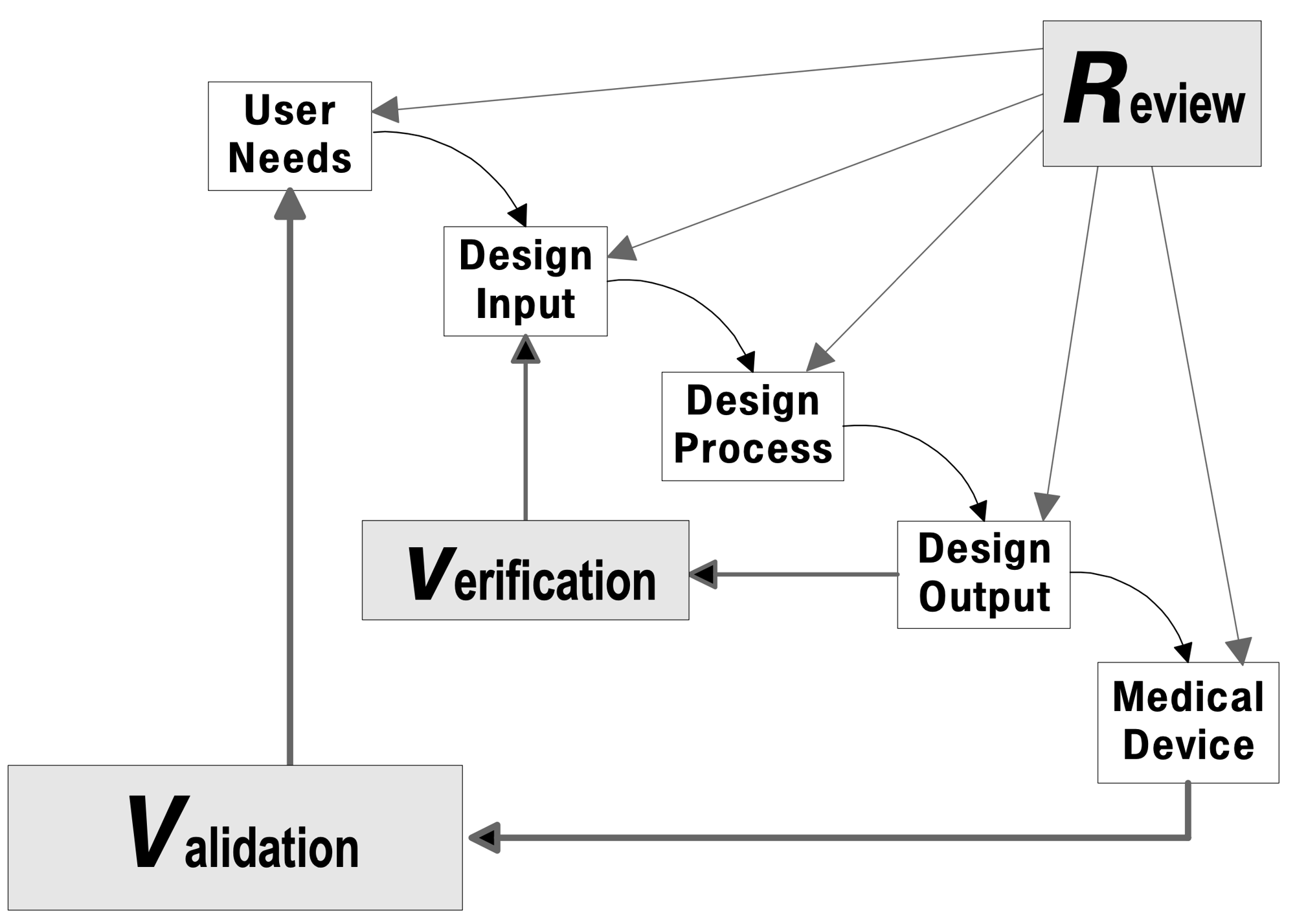
Medical device companies recognize the benefits of Agile development methods, while having concerns about its suitability for the safety-critical, regulated world of medical device software and systems.
The Association for the Advancement of Medical Instrumentation (AAMI) addressed these concerns by publishing "AAMI TIR45:2023 Guidance on the use of AGILE practices in the development of medical device software." As one of the lead authors of both the first edition published in 2012 and the second addition published in 2023, and as the lead instructor for AAMI-sponsored courses, Agile Quality Systems knows how to apply Agile Software Development in the medical device domain, getting the advantages of Agile while meeting regulatory expectations.

While compliance is required, what matters more is the guidance that regulations and standards provide for product developers, helping them to develop safe and effective products. Agile Quality Systems knows how to apply the rigor and discipline of process in practical ways that help development teams focus their energy on creating robust designs.

Software Online Agile Regulatory Fundamentals Training for today's medical software engineer. This is a 16 course training program that has been designed to maintain the team's agility while:
©2026 Agile Quality Systems. All rights reserved. Privacy Policy